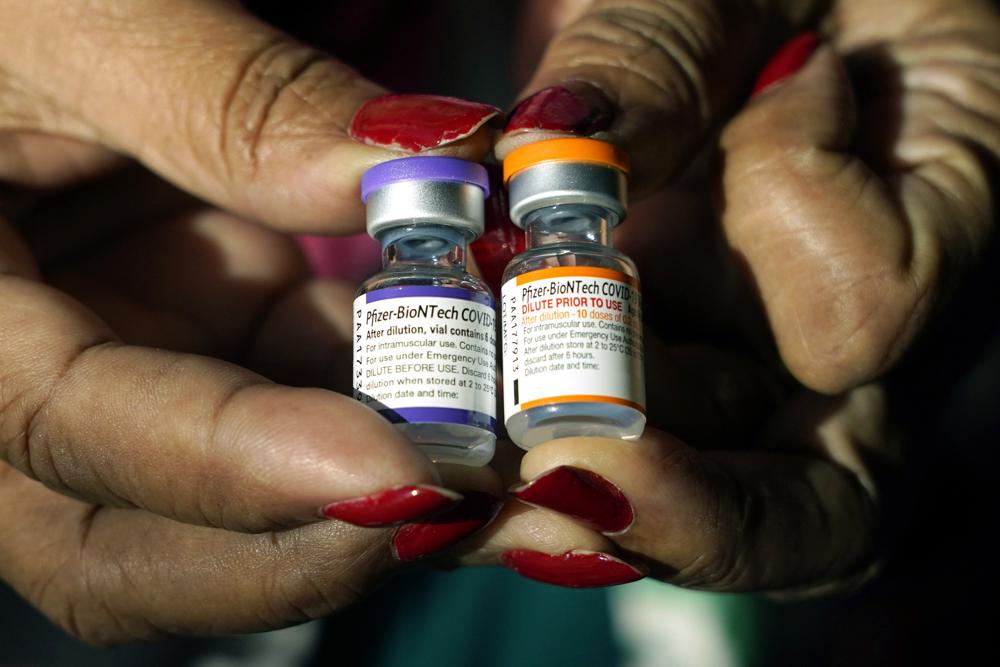
FILE – A nurse holds a vial of the Pfizer COVID-19 vaccine for children ages 5 to 11, right, and a vial of the vaccine for adults, which has a different colored label, at a vaccination station in Jackson, Miss., Tuesday, Feb. 8, 2022. U.S. regulators authorized a COVID-19 booster shot for healthy 5- to 11-year-olds on Tuesday, May 17, 2022, hoping an extra vaccine dose will enhance their protection as infections once again are on the rise. (AP Photo/Rogelio V. Solis, File)
U.S. regulators on Tuesday authorized a COVID-19 booster shot for healthy 5- to 11-year-olds, hoping an extra vaccine dose will enhance their protection as infections once again creep upward.
Everyone 12 and older already was supposed to get one booster dose for the best protection against the newest coronavirus variants — and some people, including those 50 and older, can choose a second booster.
The Food and Drug Administration’s authorization now opens a third shot of Pfizer’s vaccine to elementary-age kids, too — at least five months after their last dose.
There is one more hurdle: The Centers for Disease Control and Prevention must decide whether to formally recommend the booster for this age group. The CDC’s scientific advisers are scheduled to meet on Thursday.
Pfizer and its partner BioNTech make the only COVID-19 vaccine available for children of any age i n the U.S. Those ages 5 to 11 receive one-third of the dose given to everyone 12 and older.
Whether elementary-age children need a booster has been overshadowed by parents’ outcry to vaccinate even younger tots, those under 5 — the only group not yet eligible in the U.S. Both Pfizer and rival Moderna have been studying their shots in the youngest children, and the FDA is expected to evaluate data from one or both companies sometime next month.
For the 5- to 11-year-olds, it’s not clear how much demand there will be for boosters. Only about 30% of that age group have had the initial two Pfizer doses since vaccinations opened to them in November.
But Pfizer’s vaccine “is effective in helping to prevent the most severe consequences of COVID-19 in individuals 5 years of age and older,” said FDA vaccine chief Dr. Peter Marks ”A booster dose can help provide continued protection against COVID-19 in this and older age groups.”
In a small study, Pfizer found a booster revved up those kids’ levels of virus-fighting antibodies — including those able to fight the super-contagious omicron variant — the same kind of jump adults get from an extra shot.
While the coronavirus is more dangerous to adults than to children, youngsters can get severely ill — and more than 350 children ages 5 to 11 have died, according to CDC’s count.
Adding to public confusion, the CDC estimates 3 out of every 4 U.S. children of all ages have been infected with the coronavirus since the pandemic’s start — many of them during the winter omicron wave. Still, health authorities urge vaccination even in people who’ve previously had COVID-19, to strengthen their protection.
With subtypes of omicron now spreading, the U.S. is averaging about 91,000 cases reported a day, compared to about 57,000 just two weeks ago. That’s a small fraction of the infections seen during the brutal winter surge — but experts also say it’s a vast undercount as testing has dropped and at-home tests often aren’t reported.
Vaccination may not always prevent milder infections, especially as omicron and its siblings are better than some prior variants at slipping past those defenses. But health authorities agree the vaccinations continue to offer strong protection against the worst outcomes of COVID-19, including hospitalization and death.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education.
Copyright 2021 Associated Press. All rights reserved.
Source: https://apnews.com/article/covid-boosters-kids-fda-approval-d0f83fd5508218e1541e3a2db3172ba5