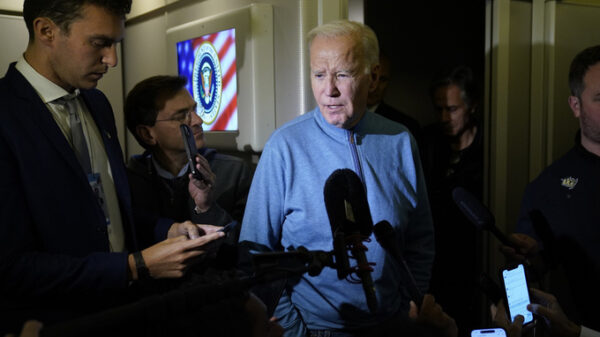
Michele Hall stands with her husband, Doug, in their backyard Thursday, June 24, 2021, in Bradenton, Fla. Hall, 54, diagnosed with early Alzheimer’s last year, calls the new drug Aduhelm “the first tiny glimmer of hope” she’ll get more quality time with her husband and their three adult children. (AP Photo/Steve Nesius)
In the weeks since a new Alzheimer’s drug was approved, hopeful patients have bombarded Dr. Alireza Atri with calls and emails about a treatment that has sparked both excitement and skepticism.
They want to know if the drug might be right for them. Like many physicians, Atri has no easy answers.
“It’s not a simple yes or no,” said the neurologist at the Banner Sun Health Research Institute in Arizona.
It probably won’t be for a while. Doctors across the country are still trying to figure out who should receive the drug called Aduhelm, which, at best, slows the fatal disease marginally. Other drugs for Alzheimer’s disease only temporarily ease symptoms like memory problems, insomnia and depression.
While some clinics have already started giving the drug, many providers say it will take weeks or months before they are ready. Insurers — including the biggest bill payer for this drug, Medicare — still need to determine which patients to cover for a treatment that could cost more than $50,000 a year. And doctors worry that emotions will affect decisions by patients and families in seeking the drug.
“People are desperate. It’s a really horrible disease,” said Stanford University’s Dr. Michael Greicius.
Karl Newkirk hopes to start taking Aduhelm if his doctor gives the OK because he doesn’t see any other treatments worth trying.
“It looks like the only star in the sky,” said the 80-year-old Sarasota, Florida, resident, who has early-stage Alzheimer’s.
Newkirk’s doctor confirms he’s a good candidate for the drug. While the retired technology consultant is still fit enough to ride roller coasters with his grandchildren at nearby Busch Gardens, he struggles with short-term memory loss. He wants to try Aduhelm, even though he’s aware of the drug’s limits.
Michele Hall, 54, of Bradenton, Florida, also is eager to discuss the drug at her next appointment with an Alzheimer’s specialist.
A former government attorney, Hall had to quit her job after struggling with once-simple tasks like spelling, public speaking and remembering deadlines. She was diagnosed with early Alzheimer’s last November by doctors at the Mayo Clinic.
Hall calls Aduhelm “the first tiny glimmer of hope” that she’ll get more quality time with her husband and their three adult children.
“When you get that diagnosis, you really wake up every morning and go ‘Here I am, it’s ticking away and I’m just waiting,’” she said. “Well, now you have something to look forward to.”
Biogen’s Aduhelm is the first Alzheimer’s medication in nearly 20 years. Its Food and Drug Administration approval earlier this month triggered a swift backlash from many experts, including the agency’s own outside advisers who had warned that its supposed benefit relied on flimsy data. Three resigned over the FDA’s decision.
Aduhelm does not reverse mental decline. It only slowed it in one study that was marred by hard-to-interpret results. The data were so murky that the FDA ultimately granted the drug conditional approval based on a different measure: it’s ability to get rid of harmful clumps of plaque in the brains of patients with early forms of the disease.
The FDA approval isn’t limited to those early patients. Anyone with Alzheimer’s — at least theoretically — could get prescribed the drug. But advocacy groups like the Alzheimer’s Association and many doctors say the focus should be patients with an early diagnosis, like those helped in the study.
“I don’t want to see people pull their mothers from nursing homes to get this treatment,” said Dr. Babak Tousi, a Cleveland Clinic geriatrician who consulted with Biogen and helped run one of the testing sites for Aduhelm.
Safety will be a key consideration, according to Dr. Ronald Petersen at the Mayo Clinic, which is coming up with its own use guidelines for the drug.
“We want to be conservative here,” said Petersen, an Alzheimer’s specialist who has consulted with most major drugmakers in the field, including Biogen.
About 40% of patients getting the full drug dose in Biogen’s studies had swelling or tiny bleeds in the brain. While the side effects usually resolved, in rare cases they led to more severe bleeding that could potentially cause brain injury or other dangerous complications.
Monitoring patients on the drug involves regular brain scans. That’s on top of a different type of scan to tell if patients have the brain plaque targeted by the drug. Running all those tests could easily approach $10,000 the first year, according to physicians.
Insurers will likely require prior approval of those scans, which could delay care. And depending on coverage, patients still might be liable for thousands of dollars annually from the scans and treatments due to deductibles and other out-of-pocket costs.
Biogen says about 900 sites in the U.S. have the equipment and expertise to immediately begin giving the drug, which requires monthly IVs.
The private Michigan Institute for Neurological Disorders has already started treating early-stage Alzheimer’s patients. The institute said it will pick up most of the cost if an insurer ultimately denies coverage “because it’s a therapy we believe in,” a spokeswoman said.
Meanwhile, Stanford’s Greicius, a neurologist and Alzheimer’s specialist, has no plans to prescribe Aduhelm.
“I don’t think there’s sufficient evidence that it works, and there’s plenty of evidence that it can harm patients,” he said.
He said he plans to lay out a “compelling and compassionate” case for why he doesn’t want to give patients the medicine. But he worries that some patients may simply turn to a doctor who will provide it.
The Cleveland Clinic’s Tousi said talking about expectations, cost and the side effects may counter some emotional pressure to give the drug to patients not suited for it.
But part of the challenge is family members often think a patient is in an earlier stage of the disease than they actually are. He said they have to understand that the medication will not bring someone back to who they were.
“What we wish cannot always be translated to real life,” he said.
One likely consequence of Aduhelm’s approval is earlier screening and diagnosis for Alzheimer’s, a longtime aim of those who study the disease, given that it develops slowly over years or decades.
But an earlier diagnosis combined with Aduhelm’s incremental benefit may simply prolong the hardships of caring for someone with Alzheimer’s.
“It could turn out that it actually increases your caregiver burden if it’s just slowing things down a little bit,” said Dr. Suzanne Schindler of Washington University in St. Louis. “I think it’s going to be really disappointing for a lot of people.”
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Copyright 2020 Associated Press. All rights reserved.
Source: https://apnews.com/article/business-science-health-2e1e0d9354502c8e3b208a8c5589fc57