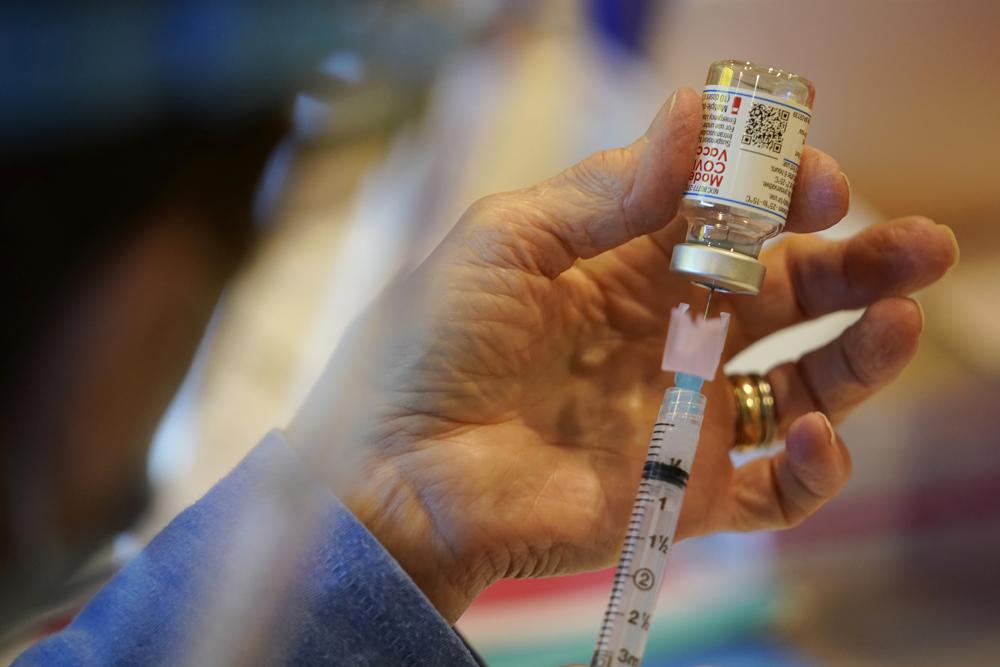
FILE – In this Dec. 29, 2020, file photo a Chester County, Pa., Health Department worker fills a syringe with Moderna COVID-19 vaccine before administering it at the Chester County Government Services Center in West Chester, Pa. Moderna said Monday, Oct. 25, 2021 that a low dose of its COVID-19 vaccine is safe and appears to work in 6- to 11-year-olds. It is the second U.S. vaccine aimed at eventually being offered to children. (AP Photo/Matt Slocum, File)
Moderna said Monday that a low dose of its COVID-19 vaccine is safe and appears to work in 6- to 11-year-olds, as the manufacturer joins its rival Pfizer in moving toward expanding shots to children.
Pfizer’s kid-size vaccine doses are closer to widespread use. They are undergoing evaluation by the Food and Drug Administration for youngsters in nearly the same age group, 5 to 11, and could be available by early November. The company’s vaccine already is authorized for anyone 12 or older.
Moderna hasn’t yet gotten the go-ahead to offer its vaccine to teens but is studying lower doses in younger children while it waits.
Researchers tested two shots for the 6- to 11-year-olds, given a month apart, that each contained half the dose given to adults. Preliminary results showed vaccinated children developed virus-fighting antibodies similar to levels that young adults produce after full-strength shots, Moderna said in a news release.
The study involved 4,753 children ages 6 to 11 who got either the vaccine or dummy shots. Moderna said that like adults, the vaccinated youngsters had temporary side effects including fatigue, headache, fever and injection site pain.
The study was too small to spot any extremely rare side effects, such as heart inflammation that sometimes occurs after either the Moderna or Pfizer vaccines, mostly among young men.
Moderna released no further details and hasn’t submitted its data to a scientific journal but said it plans to share the interim results with the FDA and global regulators soon. The study is still going on, and the company cannot calculate the vaccine’s effectiveness in actually preventing infections in children unless there are sufficient COVID-19 cases to compare rates between vaccinated and unvaccinated participants.
The FDA hasn’t yet ruled on the company’s application to expand its vaccinations to 12- to 17-year-olds, although some countries have cleared Moderna’s shots for adolescents.
But the U.S. is expected to begin vaccinating children under 12 sometime next month, if the FDA clears low doses of the Pfizer vaccine for 5- to 11-year-olds. Pfizer reported last week that its kid-size doses proved nearly 91% effective at preventing symptomatic COVID-19 in that age group, even as the extra-contagious delta variant was spreading widely.
FDA’s advisers will weigh Pfizer’s evidence in a public meeting Tuesday. If the agency authorizes Pfizer’s kid shots, the Centers for Disease Control and Prevention the following week is set to recommend who should receive them.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education.
Copyright 2021 Associated Press. All rights reserved.