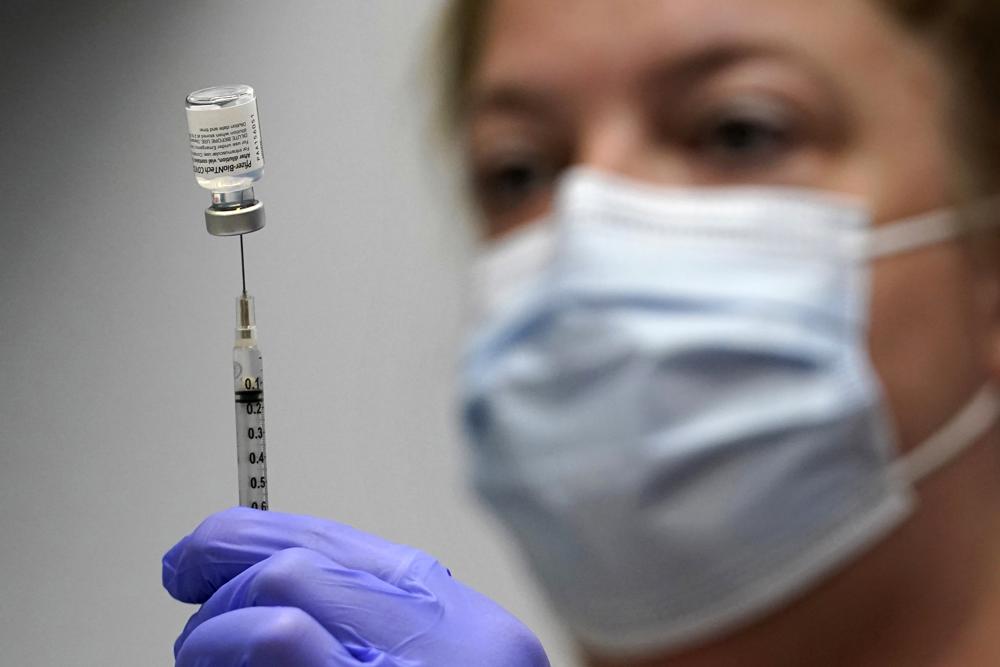
FILE – In this March 2, 2021, file photo, Hollie Maloney, a pharmacy technician, loads a syringe with Pfizer’s COVID-19 vaccine at the Portland Expo in Portland, Maine. The Biden administration’s embattled plan to dispense COVID-19 booster shots to most Americans faced its first key hurdle Friday, Sept. 17, as a government advisory panel met to decide whether to recommend extra doses of the Pfizer vaccine. (AP Photo/Robert F. Bukaty, File)
WASHINGTON (AP) — The Biden administration’s embattled plan to dispense COVID-19 booster shots to most Americans faced its first major hurdle Friday as a government advisory panel met to decide whether to endorse extra doses of the Pfizer vaccine.
Scientists inside and outside the government have been divided in recent days over the need for boosters and who should get them, and the World Health Organization has strongly objected to rich nations giving a third round of shots when poor countries don’t have enough vaccine for their first.
The panel, made up of outside experts who advise the Food and Drug Administration, weighed a less than clear-cut case: While research suggests immunity levels in those who have been vaccinated wane over time and boosters can reverse that, the Pfizer vaccine is still highly protective against severe illness and death, even amid the spread of the highly contagious delta variant.
The FDA experts were scheduled to vote on one basic question: Does the evidence show that a Pfizer booster would be safe and effective for people 16 and older? In the event of a yes vote, the FDA is expected to quickly approve boosters for Pfizer’s shot.
But that is just one step in the process. The more thorny question of who should get the shots and when will be debated next week by advisers to the Centers for Disease Control and Prevention. The CDC generally adopts the recommendations of the group, which sets policy for U.S. vaccination campaigns.
Some group members have made clear they favor giving third doses to older people, nursing home residents and front-line health care workers, rather than all adults.
Separate FDA and CDC decisions will be needed in order for people who received the Moderna or J&J shots to get boosters.
Friday’s meeting came as the delta variant continues to drive U.S. cases and deaths back to levels not seen since last winter. That has given urgency to efforts by top health officials to shore up Americans’ protection against the virus.
Dr. Peter Marks, FDA’s top vaccine regulator, acknowledged the intense disagreements in opening remarks to the advisory panel.
“We know there may be differing opinions in interpreting the data,” he said. “We strongly encourage all the different viewpoints to be voiced and discussed regarding the data, which is complex and evolving.”
President Joe Biden’s top health advisers, including the heads of the FDA and CDC, first announced plans for widespread booster shots a month ago, targeting the week of Sept. 20 as an all-but-certain start date. It said boosters would be dispensed eight months after the second dose of the Pfizer and Moderna vaccines.
But that was before FDA staff scientists had completed their own assessments of the data. Some experts questioned whether Biden was breaking his own pledge to “follow the science” on COVID-19 by getting out ahead of government scientists.
Earlier this week, two top FDA vaccine reviewers joined a group of international scientists in publishing an editorial rejecting the need for boosters in healthy people. The scientists said continuing studies show the shots are working well despite the delta variant.
The editorial came two weeks after the two reviewers announced plans to retire from the FDA. One of them, Dr. Marian Gruber, addressed the American people directly in her opening remarks Friday but gave no reason for her retirement.
“All my actions and decisions over my 32-year FDA career have been grounded in science with you in mind and in the best interest of your health and safety,” Gruber said.
The Biden booster plan has raised major ethical concerns, given the dire shortages of vaccine in impoverished parts of the world. But the administration has argued that the plan is not an us-or-them choice and noted that the U.S. is supplying large quantities of vaccine to the rest of the globe.
The U.S. has already approved Pfizer and Moderna boosters for certain people with weakened immune systems, such as cancer patients and transplant recipients. And some Americans, healthy or not, have managed to get boosters on the sly by going to clinics and pretending they have yet to get their initial shots.
Pfizer was expected Friday to present data suggesting immunity from its vaccine begins to wane somewhere around six to eight months after the second dose.
The panel was also set to hear from Israeli health officials, who began offering boosters over the summer. Officials there tracked about 1 million people 60 and older and found that those who got the extra shot were far less likely to become infected soon afterward.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Copyright 2021 Associated Press. All rights reserved.