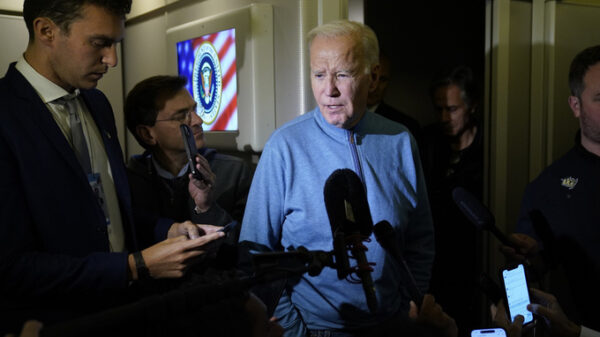
In this Feb. 12, 2019 photo, Meghan Waldron walks down the street in Boston. Waldron is a student at Emerson College with progeria, one of the world’s rarest diseases. The first treatment has been approved for progeria, Friday, Nov. 20, 2020. The U.S. Food and Drug Administration on Friday approved Zokinvy which was shown in testing to extend patients’ lives by 2 ½ years on average. (Suzanne Kreiter/The Boston Globe via AP)
The first drug was approved Friday for a rare genetic disorder that stunts growth and causes rapid aging in children, after studies showed it can extend their lives.
Kids with the genetic disorder progeria typically die in their early teens, usually from heart disease. But in testing, children taking the drug Zokinvy lived 2 1/2 years longer on average.
The U.S. Food and Drug Administration approved the capsules for progeria and a related condition.
Research on the treatment was mainly funded by the Progeria Research Foundation in Peabody, Massachusetts, with help from drug developer Eiger BioPharmaceuticals.
“This is just the first. We’ll find more and better treatments,” said Dr. Leslie Gordon, the foundation’s medical director.
Gordon, a pediatric disease researcher at Hasbro Children’s Hospital in Providence, Rhode Island, created the foundation in 1999 with her sister and husband, soon after their son Sam was diagnosed. He died in 2014 at age 17.
Just an estimated 400 people worldwide have progeria or its related condition, including 20 in the U.S. The disorder causes stunted growth, stiff joints, hair loss and aged-looking skin. Children with the disease suffer strokes and hardening of heart arteries, and die at 14 1/2 on average.
The disorder is not inherited but due to a chance gene mutation that causes a damaging buildup in cells of a protein called progerin, for which the disorder is named. The drug blocks production and accumulation of the protein, slowing its damage and the premature aging.
Until testing began in 2007, doctors could only try to ease some symptoms.
Meghan Waldron of Deerfield, Massachusetts, was diagnosed with progeria by age 2. She wasn’t growing or gaining weight and her hair was falling out. She was one of the first children to get the drug.
“Pretty soon,” she said, “there were obvious improvements.”
She started growing a little more — she’s now 3 feet, 7 inches tall — and tests showed a slowing of hardening of her arteries.
The 19-year-old Waldron backpacked in Europe alone last year after graduation from high school, where she ran track and cross country.
“My physical health is pretty good,” other than some joint stiffness, said Waldron, a sophomore creative writing student at Emerson College in Boston. “It’s just something I live with.”
She still takes the drug as part of a long-term follow-up study.
“I am so excited” about its approval, she said.
The FDA action was based on two studies in which a total of 62 kids took the drug twice a day. Their outcomes were compared with 81 untreated children around the world, matched by age and other characteristics.
The participants were followed for up to 11 years, and those who took the drug lived 2 1/2 years longer on average.
In all, four studies of the drug have been done at Boston Children’s Hospital, with 22 children and young adults taking the drug since 2010 or earlier. The oldest is 24 and has been taking it for 13 years.
Eiger, a small Palo Alto, California, drug developer, isn’t disclosing the price yet for Zokinvy, also known as lonafarnib, but it will be expensive since there are so few patients. Eiger will offer financial assistance so all patients can get it.
Zokinvy’s most common side effects were vomiting, diarrhea, nausea, abdominal pain and fatigue.
The foundation’s Gordon worked with National Institutes of Health Director Dr. Francis Collins on laboratory research that found the genetic cause of progeria in 2003.
She said research “coming up the pike” could possibly give patients “longer lives, stronger hearts and move towards a cure.”
The Associated Press Health & Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Copyright 2020 Associated Press. All rights reserved.
Source: https://apnews.com/article/aging-genetics-massachusetts-cd6a8d1658c6070b3c842d63101c89cc